Ceia unveiled the next generation Pharmaceutical Industrial detector range at Achema 2024.
Ceia the global benchmark for Industrial Metal Detection systems, had some exciting news to share at the recent Achema show held in Frankfurt.
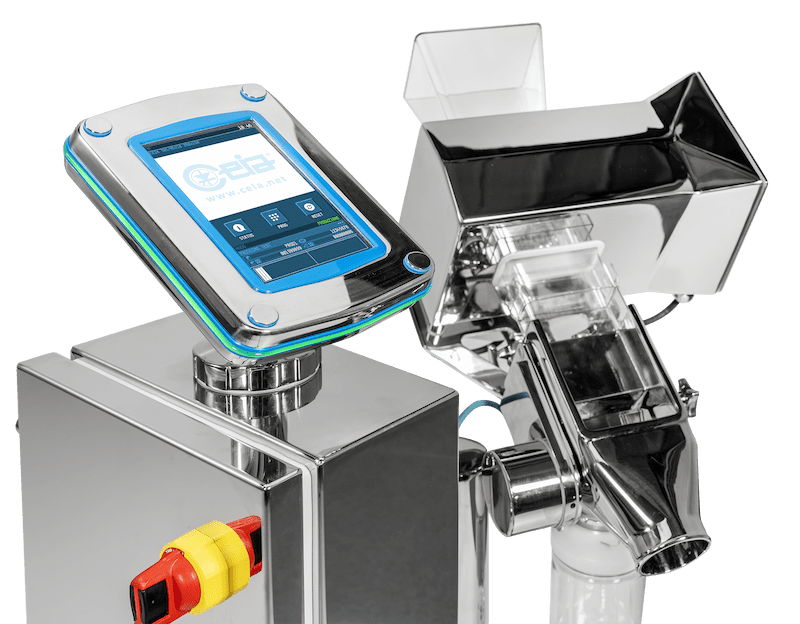
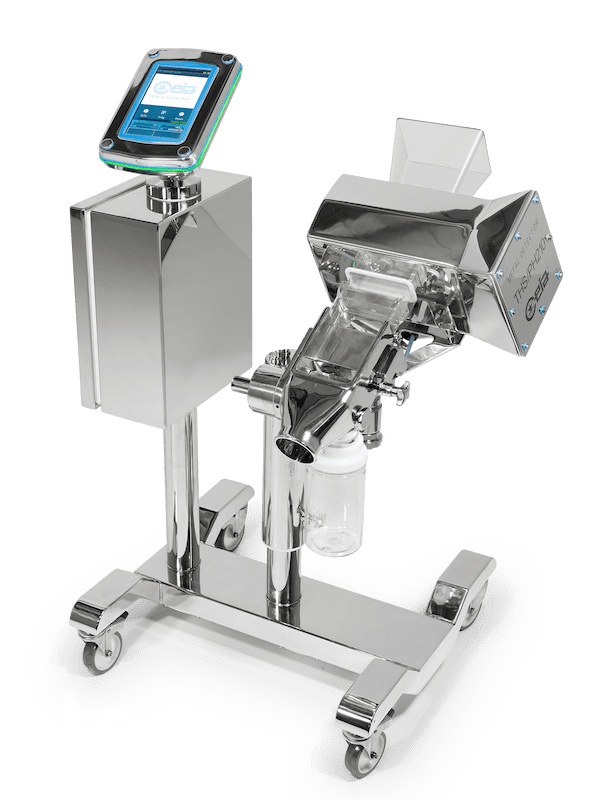
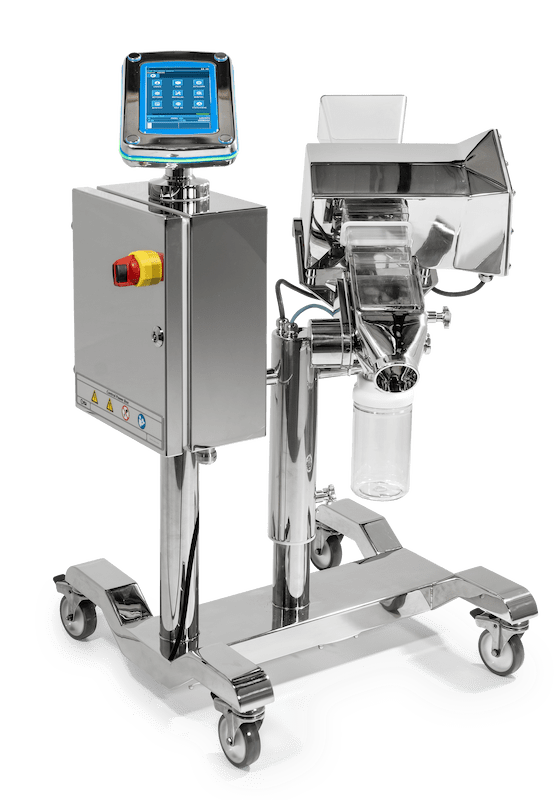
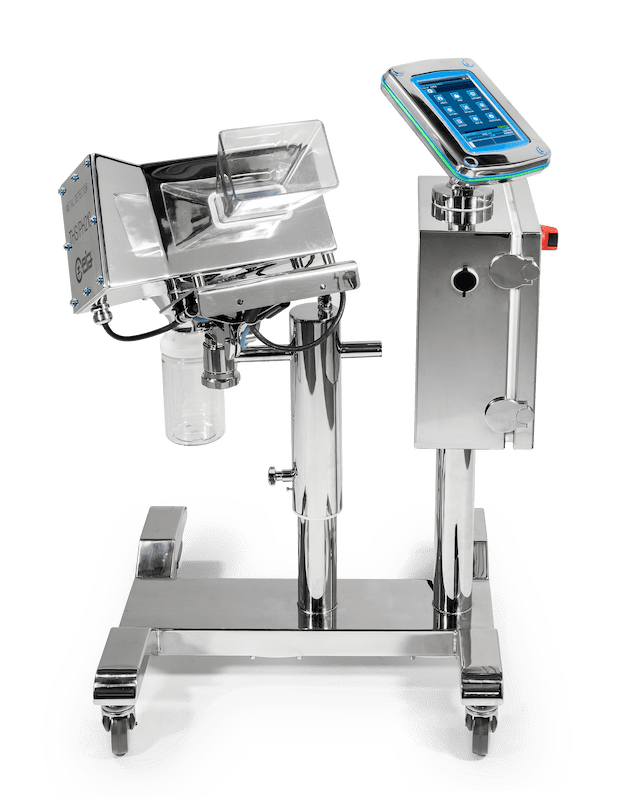
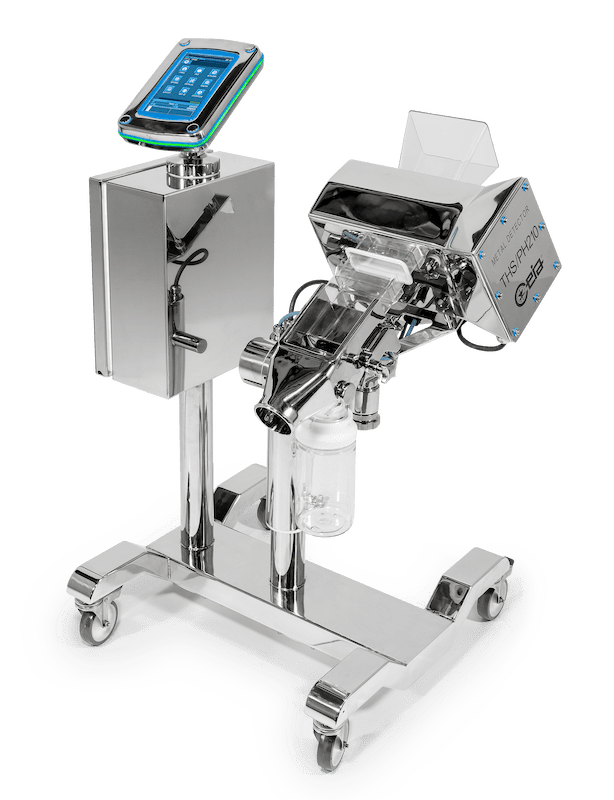
The brand new PH210 series Detector designed for the Pharmaceutical and Nutraceutical market was officially launched after many months of beta-testing with existing Key Accounts.
Ceia chose Achema, the world forum for Pharma, Biotech and Chemical markets as the best platform to introduce to the market the innovative technology.
The PH210 is the first Ceia detector to have a separate captive touchscreen designed for simplicity and easy user interface.
The Key Features for the PH21O are the improvements made to increase the level of detection capable.
- Up to 50% sensitivity improvement in terms of detectable metal volume can be seen with the new Technology.
- The system is fully constructed to AISI 316L stainless steel and the ergonomic frame design is supplied with new anti – static castors for extra stability and ease of movement.
- The main improvements centre around the embedded network controller and data logger PC. This was previously an option, but comes as standard with the PH210.
- The new detector comes with a fully digital electronic structure, new improved multi point auto learn function, auto test function and automatic QC control function to increase productivity and save on downtime
- The PH210 is fully compliant to FDA code 21 Part 11 and can store up to one million events for data integrity verification.
- For Ceia’s O.E.M partners the PH210 will have an optional embedded field bus controller for system integration. The technology uses various handshakes such as Ethernet IP, ProfNet, Profibus, Ethercat, Modbus/TCP and Profinet OPC-UA
Massimo Meacci, Sales Manager for Pharmaceutical & Nutraceutical Metal Detection Systems at Ceia commented,
“Our launch for the new PH210 has been something we have been focusing on for months and the response at the Achema show was incredibly positive. The innovative design and touchscreen have been really well received and the improvement that we can give in the level of detection is something that has really hit home to many customers !”
He added “To have already received orders on the back of the product launch at Achema and the interest we have generated over 5 days of the show proves that the wait was worthwhile “
David Hale Sales Director for MDS Ceia’s partner in the UK and Ireland for the Pharmaceutical product range commented
“Both myself and my college Pete were at the Achema show and the new detector has gained lots of positive interest, we are in the process of following the new enquires generated for the UK and Ireland and without doubt the new PH210 will be a great success based on the show’s interest “
Hale added “we are proud to announce we will officially launch the PH210 in the UK at this year’s PPMA show in September. We will have the innovative technology available for product testing, evaluation and demonstrations and would welcome our customers to visit our stand in Hall 5 stand E 10 “
For more details, please contact David Hale at david.hale@mds.org.uk , Pete Higgins at pete.higgins@mds.org.uk or via the our contact page or Ceia www.ceia.net